Redefining Pharmacokinetic Analysis: Speed, Precision, and Automation
Empowering Scientists with Seamless, Automated, and Scalable PK Analysis
WHO WE ARE
Aplos: Simplifying Data Analysis for Smarter Insights
The name "Aplos" (Greek for "simply") embodies our mission: making data analysis straightforward and accessible. At Aplos Analytics, we believe that with the right tools, data analysis should be easy to understand and perform. Our goal is to develop software that simplifies the work of data analysts, enabling them to dedicate more time to interpreting results and delivering valuable insights.
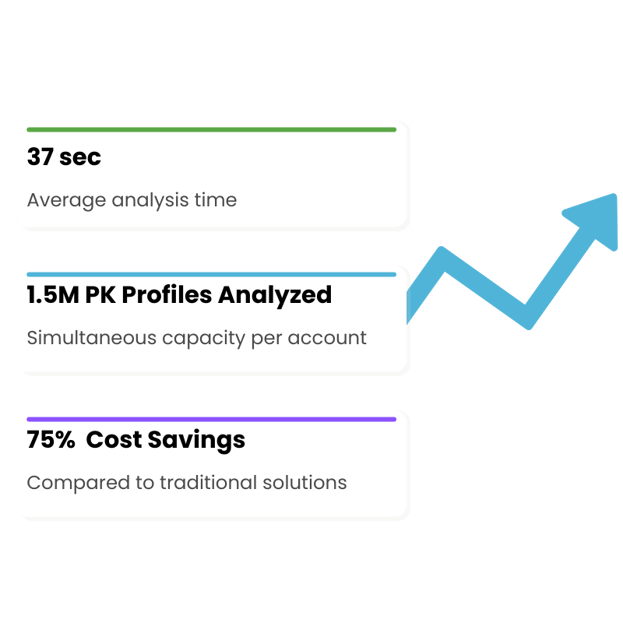

Get up and running with pharmacokinetic analysis in minutes, not hours
Streamline your pharmacokinetic analysis with Aplos NCA—no coding, no complex setup. Simply upload your data, run the analysis, and get precise results in under a minute, saving you hours of manual work
PEOPLE