Having the right tools to manage and analyze complex datasets is crucial for the success of various industries, especially Pharma and Biotechnology. Aplos NCA is a secure, cloud-based API for the calculation of PK parameters that stands out as a comprehensive solution designed to simplify and enhance the data analysis workflow.
Continuously evolving with new features, Aplos NCA supports multiple datasets formats and ensures that users can leverage their current data formats efficiently to achieve accurate and reliable results.
In this blog, we will explore current industry topics, and highlight exciting upcoming updates of Aplos NCA, that will further empower your analytical capabilities.
Software Features:
As we mentioned in the beginning of this article, Aplos NCA supports a wide range of dataset formats, enhancing integration with existing workflows.
The software accepts comma-separated variable (.csv), text-delimited (.dat), and SAS files (both .xpt and .sas7bdat). This compatibility allows users to work with diverse datasets effortlessly. Regardless of the format, Aplos NCA converts all files to .csv format before analysis, ensuring consistency.
The software handles text-delimited files using various delimiters such as pipe (|), colon (:), space, and tab. It also supports SAS Transport files (versions 5 and 8) and native SAS dataset files (.sas7bdat).
Upon downloading the results, users receive both the original format and the converted .csv file, facilitating easy verification of the conversion process.
Industry Interest Topics
In 2023, the FDA Office of Clinical Pharmacology issued nine guidance documents, addressing various critical topics in drug development. Notably, three guidances focused on drug interactions, a complex and vital area in polypharmacy management.
The FDA provided final recommendations on drug interaction studies involving oral contraceptives, therapeutic proteins, and acid-reducing agents. These documents aim to guide the industry in ensuring that drug development results adequately support the desired labeling for new drug applications. The FDA's proactive approach in releasing these guidances underscores the importance of managing drug interactions effectively to enhance patient safety and treatment efficacy.
While these guidance documents highlight a variety of methods to identify and quantify drug-drug interactions, the preferred method is to conduct a clinical study in healthy volunteers. Aplos NCA's robust data handling capabilities ensure that researchers can efficiently manage the extensive data required for such comprehensive studies and facilitates adherence to these guidelines, enabling scientists to focus on critical analysis without worrying about data compatibility issues.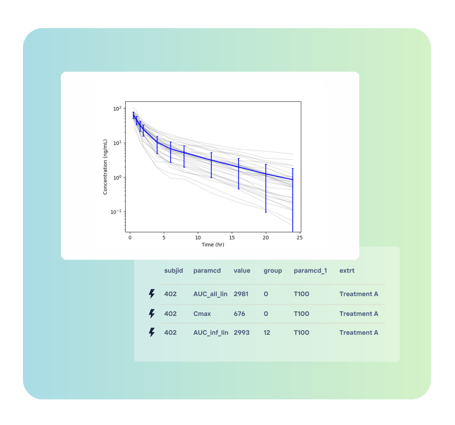
Upcoming Features
Aplos NCA is set to introduce several exciting new features in the coming months, designed to enhance collaboration and analytical capabilities for organizations. One of the key updates is the launch of team and enterprise accounts. These accounts cater to organizations requiring data sharing among multiple analysts. They will feature administrative roles for user management, data and results collaboration, and combined allocation limits.
Additionally, these accounts will support data isolation in different geographic regions, ensuring compliance with organization and country-level data storage requirements. Companies can also centralize analyses by granting vendors access to their Aplos NCA instance, maintaining control over data and results.
Another significant upcoming feature is the ability to analyze sparse datasets like those commonly found in toxicology and pre-clinical studies. In these studies, the PK parameters are derived from mean concentration-time data from multiple animals. Thus the input dataset includes individual concentration values that have to be processed to create a mean profile before calculating the relevant PK parameters. Aplos NCA will support this sparse sampling methodology with no need for pre-processing the analysis dataset.
These enhancements are meant to streamline workflows and expand the analytical capabilities of Aplos NCA, providing users with more flexibility and control in their research endeavors.
As the landscape of drug development and data analysis evolves, Aplos NCA continues to set the standard for data analysis in clinical research by providing versatile and user-friendly tools that integrate seamlessly into existing workflows.
Embrace the future of data analysis with Aplos NCA and stay ahead in your research and development endeavors!
Interested in learning more about how Aplos can help you? Contact us today to discover how we can support your goals!